Spodumene from San Diego Co., California
Spodumene from San Diego Co., California
by Waldemar T. Schaller
With Pala Presents, we offer selections from the library of Pala International’s Bill Larson, who shares with us some of the wealth of information in the realm of gems and gemology. The following article was published by the University of California in the Bulletin of the Department of Geology, Vol. 3 (1902–1904), No. 13, pp. 265–275, Plates 25–27.
This article is remarkable because immediately after it was written, an announcement was made that the subject material had been given a name: kunzite.
Contents
Beautiful transparent spodumene of deep amethystine purple, rose and magenta colors, has so far been found in two localities in southern California. The one from which most specimens have been obtained is situated about two miles north of Pala, San Diego Co., and this locality was visited by the writer during the past summer. The other place of occurrence is somewhere in the San Jacinto mountains, probably not far from Cuahuilla, Riverside Co., which is about twenty-five miles from Pala. It is probable that with further exploration other localities will be found in the Smith mountains, San Diego Co. and also in the San Jacinto mountains, Riverside Co.
The formation in which these fine crystals are found at the Pala locality consists of a pegmatite dike, dipping westerly at a low angle, perhaps 20°. It is more or less broken, and, as a whole, seems to form the surface of much of the slope of the hill, on which it occurs. The dike is rather broad, but irregular in its present shape, and has a thickness of probably not more than thirty feet. Plate 25 is a photograph of the locality, looking north-west.
So far as the mining developments have shown, only a small portion of the dike is rich in lithia minerals. Ordinarily, the dike is a coarse muscovite granite, the orthoclase and quartz predominating, containing many rounded prisms of black tourmaline, with broken ends. Lepidolite occasionally seems to replace the muscovite and when it does, red, blue and green tourmalines replace the black variety. It is with these gem tourmalines that the spodumene occurs. While the tourmaline and lepidolite are frequently inclosed in the quartz and feldspar, no such inclusions of spodumene have been found. The latter mineral always occurs associated with the other minerals, but never penetrating them or penetrated by them. It occurs in pockets and these facts seem to indicate that the formation of the spodumene is later and not coincident, in time of formation, with the tourmalines and with the dike.
The dike cuts across the large intrusion of dark rock occurring at Pala and briefly mentioned by Dr. H. W. Fairbanks. [1] This large body of dark rock, several miles across, is surrounded on all sides by granite. It is in this same body of rock, and hardly a mile from the spodumene locality, that the well known lepidolite mine is situated from which so many specimens of the fine-grained white lepidolite with the radiating groups of rubellite have been obtained. This large body of lepidolite occurs in a similar pegmatite, having the same general strike and dip as the dike carrying the spodumene, which mineral has not as yet been found in the lepidolite mine.
The dark rock forming the footwall of the dike in which the spodumene occurs, is a diorite, consisting of hornblende, a plagioclase, and (subordinate) orthoclase with accessory magnetite and apatite.
The hornblende is common green hornblende, with usually ragged outline, and possesses normal optical properties. b = b, c ∧ c = 16°. The pleochroism is a = light yellow to light olive green, b = dark olive green, c = sea green to blue green. The absorption is c > b > a. The triclinic feldspar predominates as is shown by the albite twinning lamellae present. Symmetrical extinction angles gave values from 30° to 35°, making this feldspar labradorite. The orthoclase occurs sparingly in nearly square sections and is distinguished from the labradorite by its lower relief and birefringence. From its structure and mineral contents the rock is evidently a basic diorite containing some orthoclase. From a description of the other locality, the mode of occurrence of the spodumene there, seems to be similar to that at Pala, being a dike in “blue granite” (the name locally given to the diorite).
Only the prismatic zone is well defined on the specimens seen by the writer; both ends of the crystals are rounded off, and any forms occurring there are practically undeterminable. On one of the smaller crystals, the two forms s = {121} and p = {112} may possibly be present. [2] The
| Dana | Gdt. | |
| p q | = | — (p/2 + 1).q/2 |
| —2(p + 1).2q | = | p q |
measurement of these two forms, with the two circle goniometer, gave the following results:
| Measured | Calculated | |||
| φ | ρ | φ | ρ | |
| s = 12 = {121} | 37° 14' | 73° 33' | 38° 46' | 72° 56' |
| p = —1/2 = {112} | 21° 05' | 31° 57' | 20° 05' | 34° 04' |
The forms in the prism zone, however, can easily be determined. They are
| b = 0∞ = {010} | n = ∞3 = {130} |
| a = ∞0 = {100} | A = ∞5/3 = {350} |
| m = ∞ = {110} | l = 3/2 ∞ = {320} |
The measurement of the angles of these forms with those calculated, are given in the following table.
| Measured | Calculated | |||
| φ | ρ | φ | ρ | |
| b = 0∞ = {010} | 0° 06' | 90° 00' | 0° 00' | 90° 00' |
| a = ∞0 = {100} | 90° 00' | " | 90° 00' | " |
| m = ∞ = {110} | 43° 30' | " | 43° 30' | " |
| n = ∞3 = {130} | 17° 06' | " | 17° 33' | " |
| A = ∞5/3 = {350} | 29° 50' | " | 29° 39' | " |
| l = 3/2∞ = {320} | 55° 01' | " | 54° 54' | " |
The form A = ∞ 5/3 = {350} is new for spodumene and occurs but once, as a small face.
The form n = ∞ 3 = {130} was measured by means of a wax impression, as the crystal on which it occurs is too large for measurement with the reflection goniometer.
The unit prism is always present, and measurements of ten faces gave the following values:
| φ angle on m = ∞ = {110} | |
| 43° 30' | 43° 31' |
| 43° 29' | 43° 28' |
| 43° 36' | 43° 26' |
| 43° 24' | 43° 33' |
| 43° 33' | 43° 30' |
| Av. = 43° 30' | |
This value agrees with the one given by Dana in his System, but varies somewhat from the angle obtained by Brush and Dana [3] on cleavage faces of the Branchville, Conn., spodumene, their results giving 43° 36.5'.
The interfacial angles on the large crystals were measured with a hand goniometer and the averages of these measurements are shown in the following table.
| Measured | Calculated | |
| (110) : (110) | 93° 18' | 93° 00' |
| (110) : (110) | 87° 24' | 87° 00' |
| (110) : (010) | 44° 00' | 43° 30' |
| (110) : (100) | 46° 36' | 46° 30' |
| (100) : (010) | 89° 30' | 90° 00' |
The unit prism, while always present is not always equally developed in its four faces, two parallel faces being frequently much larger than the other two. The crystal thus presents a tabular appearance.
The orthopinacoid is rather frequently present, though occasionally it is very narrow and rounded to such an extent as to render it difficult to definitely decide if it be present or not. Then again, it may be very broad making the crystal tabular. A marked feature of the orthopinacoid is that it is always deeply furrowed vertically.
The clinopinacoid is not of frequent occurrence, though it has been noted a number of times, from a narrow face, less than a millimeter wide to one almost a centimeter in width. Fig. 2, Plate 26, shows the crystal having the broadest clinopinacoid. From left to right the faces in the prism zone, on this crystal, are (130) (very narrow), (010), (110), (100) (furrowed). The orthopinacoidal faces are the only ones striated.
Three habits are noticed in these crystals, depending on the relative size of the faces in the prismatic zone.
The most common habit and the one that is more or less confined to the smaller crystals, is a tabular form resulting from the inequality in size of the prism faces. Other faces, such as the pinacoids are usually absent from crystals of this type.
The second most frequent type is where the orthopinacoid is very large and the crystals become tabular parallel to this form. This habit seems to be restricted to the larger crystals.
In the third habit, all three forms, the prism and the two pinacoids are equally developed and the crystal becomes octagonal in shape. This habit is of rare occurrence.
Plate 26 is a photograph of seven crystals. Crystal 6 has the first habit, crystals 4 and 7 have the second habit, and crystal 2 has the third habit.
A very marked feature of these crystals is the profusion of natural etch figures which thickly crowd all of the natural faces of the crystals, except the orthopinacoid. Even cleavage (prismatic) pieces frequently show them.
On the faces of the unit prism they are especially thick as can be seen in Fig. 6, Plate 26, which is a crystal tabular to a prism face. The etch figures are usually triangular in shape and vary in size from a maximum length of about three millimeters and width of one millimeter to ones of microscopic size. Not infrequently there will be several smaller ones in the base of a larger one.
Occasionally a long string of the figures will run across a prism face, in an approximately horizontal direction.
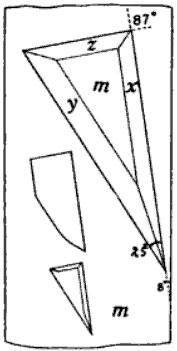
The orientation of these triangular pits, with reference to the crystallographic directions, varies somewhat but, in general, is fairly constant and is shown in Fig. 1. The position of these figures on the four prism faces is shown in Fig. 2. It is noticed that they always point away from the orthopinacoid and the angle nearly 90° is nearest the clinopinacoid. Rarely the triangle passes into a trapezium by the addition of a fourth side, as is shown in the middle figure of Fig. 1; very few of the edges are perfectly straight, being more or less rounded, but they are mostly all drawn straight.
Fig. 1, Plate 27, shows a photomicrograph of the common appearance of these etch figures—a large number of imperfect ones closely crowded together. Fig. 2 shows a view of the best group of etch figures that could be found on any of the crystals. It represents the face (110) while the one to the left of it represents the face (110).
A detailed study of these etch figures shows that they consist of four faces: three side faces and a base. Fig. 1 gives in detail a view of one of these pits. The bottom or the face lettered m is parallel to the unit prism. The face x corresponds to the prism {320}, the face y to {8.14.3} or {351}, and the face z to {11.10.3}. The measurements on which these determinations are based, are as follows:
| Measured | Calculated | ||||
| φ | ρ | φ | ρ | ||
| m | = ∞ = {110} | 43° 24' | 90° 00' | 43° 30' | 90° 00' |
| x | = 3/2∞ = {320} | 55° 49' | " | 54° 54 | " |
| 55° 40' | |||||
| 56° 09' | |||||
| av. | 55° 53' | ||||
| y | = 8/3 14/3 = {8.14.3} | 34° 33' | 81° 44' | 34° 20' | 82° 04' |
| 34° 44' | 82° 19' | ||||
| 32° 33' | 82° 04' | ||||
| av. | 33° 57' | 82° 02' | |||
| = 35 = {351} | " | " | 35° 01' | 82° 04' | |
| z | = —11/3 10/3 = {11.10.3} | 40° 44' | 80° 20' | 40° 15' | 79° 47' |
| 40° 29' | 79° 25' | ||||
| 41° 06' | 80° 06' | ||||
| 40° 23' | 79° 22' | ||||
| av. | 40° 41' | 79° 48' | |||
For the face y the measurements are approximate for the two symbols {8.14.3} and {351} and the simpler one may therefore be chosen.
Quite frequently the faces m and x grade insensibly into one another or the face m may be entirely lacking. All of the faces are usually very much rounded, making it difficult to obtain any accurate measurements.
The symbols obtained for the faces of the etch figures do not at all agree with those determined by Dana [4] on Hiddenite.
The form z = {11.10.3} approximates the form g = {441} = {681} (Dana).
| Measured | Calculated | |||
| φ | ρ | φ | ρ | |
| 40° 41' | 79° 48' | {11.10.3} | 40° 15' | 79° 47' |
| {441} | 38° 07' | 81° 12' | ||
The forms determined by him compared with those obtained by the writer, given in Goldschmidt’s and Dana’s orientations respectively, are:
The etch figures occurring on the clinopinacoid are rhombic in shape and sometimes modified by another plane, as is shown in the upper figure in Fig. 3, which also shows the position of the etch figures with reference to the crystal. A few of the etch figures run over on the clino prism (130), the clinopinacoid here shown being the negative one. Strings of the etch figures also occur pitching in a direction opposite to that of the clinoaxis.
Fig. 3. Showing the position of etch figures on the clinopinacoid (010) and the optical orientation of the crystal.
Figures 3 and 4, Plate 27, show photomicrographs of the clinopinacoidal etch figures. It will be noticed that there are two prominent varieties: the rhombic, nearly equal in the two directions, and then a long narrow form such as is shown in Fig. 3. They are all identical, as the long narrow ones are simply distorted in one direction.The prism {320} occurs on the clinopinacoidal etch figures and is the only form determinable. A remarkable fact is that on one crystal, the etch figures penetrate the crystal, emerging on the opposite clinopinacoid. These cavities are usually partially choked up by a limonitic earthy substance.
Etch figures also cover the rounded ends of the crystals, but their relation to the structure of the crystal cannot be determined owing to the lack of definite terminal faces.
In its physical properties, with the exception of the color, the mineral does not vary from normal spodumene. The color, although rare, is not new as it has been noted on some of the spodumene from Branchville, Conn., described by Brush and Dana. [5] To quote a few words from their paper (p. 258}—“The unaltered spodumene, of a fine amethystine color,… In the better specimens the spodumene is perfectly transparent, sometimes colorless, and again of a beautiful rose-pink or amethystine-purple color.” This description fits admirably with the San Diego spodumene. One specimen is colorless while the others are of a rose-pink, magenta or amethystine-purple color.
The cleavage parallel to the unit prism is highly perfect. No parting parallel to the orthopinacoid was noticed on any of the crystals. All of the crystals are transparent and show no trace of alteration, being remarkably fresh and thus differing materially from the New England spodumene. The hardness of the mineral lies between that of quartz and beryl. An interesting observation may be mentioned here. On cutting a crystal with a carborundum saw, the mineral became strongly luminous.
The clearest transparent piece was taken for analysis and the specific gravity of it taken by suspension in water. The piece weighed about 10 grams. The specific gravity was determined as 3.189. This agrees very well with the result obtained on the pink Branchville, Conn. spodumene by Brush and Dana, namely, 3.193.
The mineral fuses easily to a clear glass coloring the flame an intense red.
The pleochroism is a very striking property of the mineral. The colors are a = magenta or amethystine-purple, b = pale pink or amethyst, c = colorless. The c ray is absolutely colorless no matter how thick the crystal is.
The average of a number of determinations of the inclination of the acute bisectrix to the vertical axis, gave, for sodium light, Bxa ∧ c = +25° 24'. That is, the acute bisectrix lies in the obtuse angle β and is nearly normal to the basal pinacoid (Dana’s orientation). The indices of refraction, α and γ were determined by the method of Duc de Chaulnes on a crystal about a centimeter in thickness. The values obtained, the average of a number of readings, are only approximate, though they agree remarkably well with the determinations of Des Cloizeaux. The results obtained by the writer for sodium light, are:
α = 1.652
γ = 1.679
This gives, as the strength of the double refraction, γ – α = .027. The results of Des Cloizeaux are:
Na α = 1.652
γ = 1.679
Many attempts were made to prepare a section of the mineral normal to the acute bisectrix but it invariably went to pieces, owing to the highly perfect prismatic cleavage.
The average of several analyses of the mineral afforded the writer the results shown in column I.
| I. | II. | ||
| SiO2 | = | 64.42 | 64.25 |
| Al2O3 | = | 27.32 | 27.20 |
| Mn2O3 | = | 0.15 | — |
| Li2O | = | 7.20 | 7.62 |
| Na2O | = | 0.39 | 0.39 |
| K2O | = | 0.03 | trace |
| Fe2O3 | = | none | 0.20 |
| CaO | = | none | — |
| MgO | = | none | — |
| Ign. | = | no loss | 0.24 |
| 99.51 | 99.90 |
In the second column is given an analysis of the pink spodumene from Branchville, Conn., analyzed by Professor S. L. Penfield. [6] Unfortunately Penfield does not mention the absence or presence of manganese. Otherwise the analyses agree very closely. The results for lithium obtained by the writer are probably a little low. Lithium was separated from the other alkalies by amyl alcohol. The soda fusion had a deep blue-green color and the solution of it in hydrochloric acid had a very decided pinkish cast, affording good qualitative tests for the presence of manganese. Its state of oxidation in the mineral is not known but the absence of any dyads makes it appear more reasonable to consider the manganese as present as Mn2O3, replacing the alumina. Iron is entirely absent, the alumina precipitate being, after ignition, pure white. Neither calcium nor magnesium could be detected. There was no loss on ignition either at a low red heat or at a more intense heat.
Since the above was written, short notices of this spodumene have appeared in Science for August 28 and September 4, and the gem has been named Kunzite. No detailed description of the mineral is given: only a few of the physical properties being mentioned. In both notes twinning structure is mentioned as characteristic of the mineral. A careful study of the position of the etch figures has convinced the writer that the specimens which form the subject of this study are untwinned.
The green spodumene, hiddenite, also occurs in this locality. The writer has recently received from there a small, pale green crystal, about 26 mm. long, 8 mm. broad and 7 mm. thick. The etch-figures on the prismatic faces show that this crystal is twinned, as some of the etch-figures are in reversed position to the others.
University of California,
September, 1903
1. Ninth Report State Mineralogist (California).
2. The orientation of the crystals in this paper corresponds to the one given in Goldschmidt’s Winkeltabellen. To change the indices to those given In Dana’s System, the following transformation symbols (Gdt. Index 3) are used.
3. Amer. Journ. Science, 1880 (3), 20, 257.
4. Amer. Journ. Science, 1881 (3), 22, 179.
5. Loc. cit.
6. Loc. cit.
Plate 25. View of the Gem Mine near Pala in which the Spodumene is found. Looking north. From a photograph by A. C. Lawson.
Plate 26. Characteristic forms of the Pala Spodumene.
Plate 27. Natural etch figures on the Pala Spodumene. I. On the prism 110. II. On the prism 110. III and IV. On the clinopinacoid 010.